Aluminum, the Bone and Brain Breaker
TL;DR:
Aluminum (Al) is neurotoxic and easy accumulates in the brain
Al also weakens connective tissue and softens bones by replacing calcium and phosphorus
Silica is Aluminum’s natural antagonist and has a remarkable ability to remove aluminum
Drinking silica water and supplementing silica is highly protective against Al
Eating high calcium/phosphorus foods like diary and supplementing magnesium provide secondary support
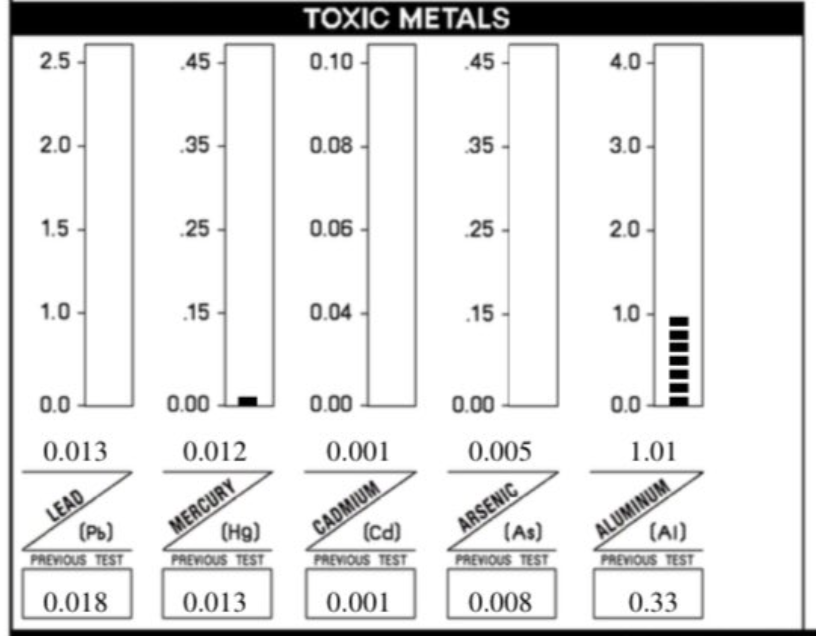
Aluminum (Al) is the third most abundant element which comprises about 8% of earth's crust, after oxygen and silicon. HTMA (Hair Tissue Mineral Analysis) practitioners commonly report that aluminum toxicity is among the most prevalent and concerning toxic metal burdens in the population. According to HTMA Experts, it is estimated that up to 93% of people tested show elevated aluminum levels in their hair, making it the most frequently detected toxic metal in clinical hair analysis. This high prevalence is attributed to widespread environmental and dietary exposure, including sources such as antiperspirants; cookware and kitchenware; artificial food coloring (FD&C Red No. 40 aluminum lake and FD&C Yellow No. 6 aluminum lake), medications that use these aluminum lakes; an adjuvant for post-2000s, dead-virus childhood vaccines, and geoengineering.
Aluminum is considered highly neurotoxic, with research and clinical observations linking elevated levels to neurological disorders such as Alzheimer’s disease, Parkinson’s disease, ALS, autism, and cognitive impairments. Aluminum tends to accumulate in brain and bone, rather than remaining in the blood.
Why is aluminum accumulate to the brain?
Aluminum (Al³⁺) exploits two primary pathways to cross the blood-brain barrier (BBB) and accumulate in the brain.
Al³⁺ binds to transferrin, the iron-transport protein, and enters the brain via transferrin receptor-mediated endocytosis at the blood-brain barrier (BBB). This pathway is critical for iron delivery to neurons, but aluminum exploits it due to its similar charge and size. While transferrin circulates everywhere, the brain’s high transferrin receptor density (e.g., in the hippocampus and cerebellum) creates a "Trojan horse" entry point for aluminum.
Unbound aluminum forms complexes with citrate, which can cross the BBB independently of transferrin. Aluminum citrate uses monocarboxylate transporters (MCTs) and system Xc⁻ (a glutamate/cystine exchanger) to bypass Tf-dependent pathways. These transporters are highly active in metabolically demanding brain regions.
Aluminum binds to amyloid-β and tau proteins, forming stable complexes that resist clearance. These aggregates are hallmarks of Alzheimer’s disease and act as "sinks" for aluminum. Al becomes trapped in neurofibrillary tangles and plaques, prolonging its retention.
Why is Aluminum attracted to bone?
Aluminum has a high binding affinity for phosphate, a key component of hydroxyapatite (bone and teeth mineral complex). It also displaces calcium, integrating itself into the bone matrix. In renal deficiency, aluminum accumulates in bone at exponential rates. Al disrupts osteoblast activity and collagen cross-linking, causing osteomalacia (soft bones). Aluminum also suppresses parathyroid hormone (PTH), impairing calcium regulation and worsening bone demineralization.
Individuals with elevated aluminum levels often show silicon deficiency on OligoScan testing due to increased physiological demand for silicon to counteract aluminum toxicity. Silicon (as silicic acid) forms stable hydroxyaluminosilicate complexes with aluminum, which are excreted via urine. This process reduces aluminum bioavailability and tissue accumulation. Oligomeric silica (non-monomeric) has 1,000,000x higher affinity for aluminum than monomeric silica, making it critical for detoxification. High aluminum exposure depletes available silicon as the body prioritizes aluminum detoxification. OligoScan reflects this increased utilization as "deficiency."
A landmark clinical trial conducted by researchers at Keele University demonstrated that daily consumption of 1 liter of silicon-rich mineral water over 12 weeks significantly reduced aluminum levels in the body by 70%.
Silica’s primary role in the body is the formation of collagen (connective tissue) and bone mineral deposition. It is most concentrated in ligaments and tendons.
Silica is essential for the synthesis and stabilization of collagen, the main structural protein in skin, bones, tendons, ligaments, and cartilage. Silica helps maintain the integrity and elasticity of connective tissues, supporting healthy skin, hair, nails, and joint flexibility. Silica is crucial for bone mineralization and strength. It cross-links collagen fibers, providing the matrix upon which calcium is deposited, and helps maintain strong, flexible bones.
The Intervertebral Disc Study showed degenerated spinal tissues showed strong correlations between elevated aluminum and silica levels, suggesting silica is mobilized to mitigate aluminum toxicity. High aluminum exposure redirects silica toward detoxification, creating a functional silica deficiency in collagen-rich tissues. This weakens connective tissues, increasing susceptibility to injuries (e.g., tendon tears, joint degeneration).
A elevated aluminum/deficient silica profile makes you high risk for musculoskeletal injuries.
How to protect yourself
Minimize exposure to the aforementioned aluminum sources
Increase intake of calcium/phosphorus foods like dairy, my favorite is greek yoghurt
Supplement magnesium to balance calcium
Supplement Orthosilicic Acid (ch-OSA) and drink high silica water
To your health,
Jonathan
This is for informational purposes only and should not replace professional medical advice. Consult with your physician or other health care professional if you have any concerns or questions about your health.